Research Interests
We take advantage of the conservation of developmental control of embryogenesis among organisms, to study the function of genes involved in congenital defects in humans by doing experiments with the frog Xenopus laevis. It is relatively easy to introduce biomolecules like antisense oligonucleotides to reduce mRNA levels, or mRNA itself to introduce or alter levels of specific protein in live oocytes or embryos. We then look for morphological and molecular changes that result. Xenopus embryos develop rapidly and are small enough to use for confocal microscopic analysis that allows both the exterior and the interior of the developing organ system to be viewed.
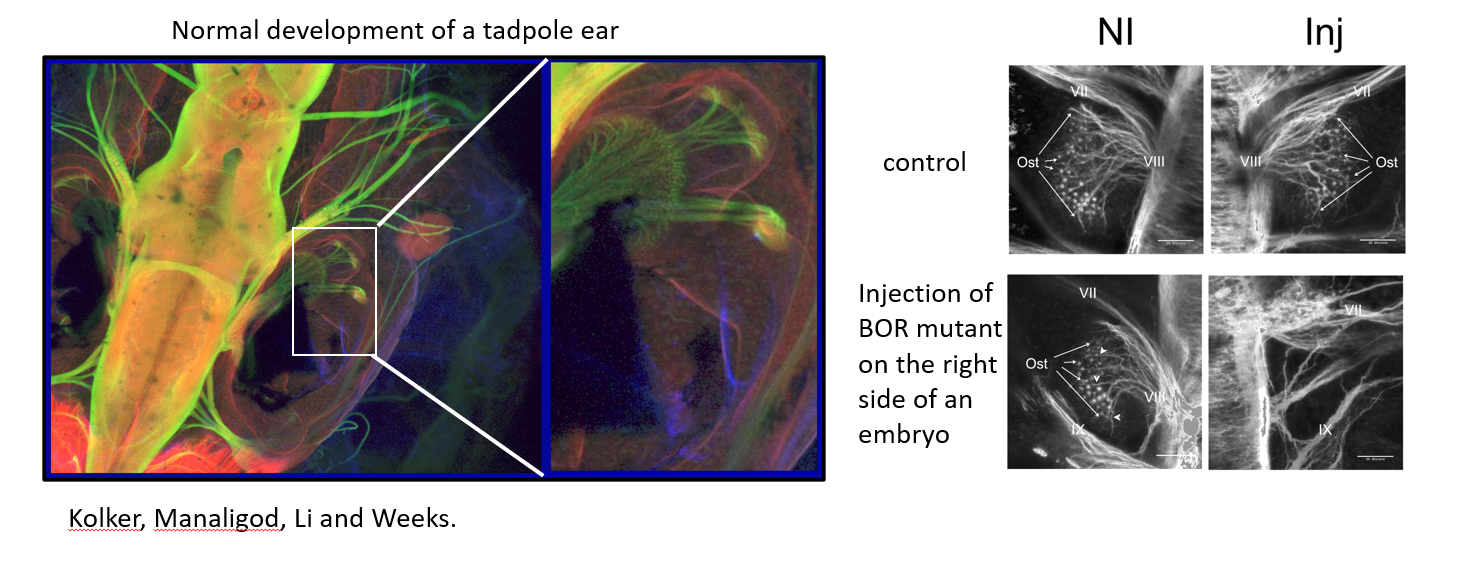
For example, the introduction of human mutations in the EYA1 protein that cause Branchio-oto-renal syndrome and leads to hearing loss was shown to disrupt the early neuronal organization of the developing ear.
Figure 1. Expression of mutant EYA1 on the right side of a tadpole alters ear development.
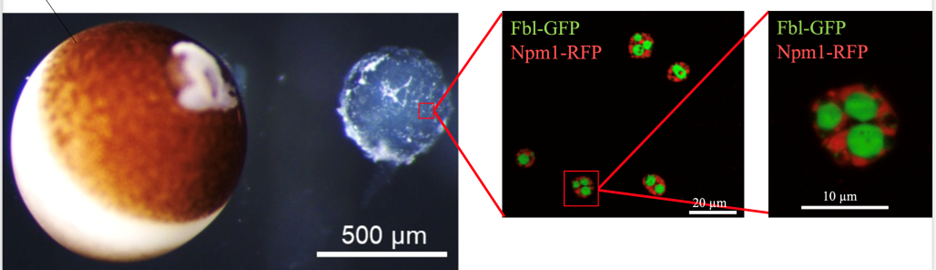
Our most current studies look at how amyloids and other protein condensates are used as part of normal development. The extracellular formation of amyloid particles is part of the pathogenesis that accompanies devastating diseases like Alzheimer's and Huntington's disease. However, it is increasingly clear that the formation of amyloids, most commonly the formation of proteins into cross-beta strand structures rather than their active, native three-dimensional structure, may be non-pathogenic and even advantageous. The best current examples of the formation of advantageous amyloid-like structures come from studies in yeast, where stress responsiveness and the ability to adapt to changing nutritional conditions are mediated through the assembly and disassembly of amyloid-like structures. The formation of amyloid-like structures is also critical for long-term memory in studies using snails and fruit flies. Other protein condensates play a role in the assembly of non-membrane-bound organelles, like nucleoli, nuclear pores and cytosolic granules involved in RNA and protein homeostasis. We now know that in oocytes the nuclear particles where transcription and RNA processing occurs contain protein condensates and amyloids as do the nucleus and cytosol of the dividing cells of the early embryo. We are investigating what proteins form amyloids and condensates and how they are assembled and disassembled as part of normal development.
Figure 2. Examining oocyte nucleolar condensates: The expression of fluorescent protein fusions of nucleolar proteins allows the study of the multi-component condensate structure of the nucleolus. Image credit: Lavering and Weeks